Abstract
Background:
Pembrolizumab (PEM), a PD-1 inhibitor, is US FDA approved for the treatment of relapsed/refractory cHL based on results of a pivotal trial demonstrating an overall response rate of 69% and complete response (CR) rate of 22%. We hypothesized that PEM monotherapy would result in a higher CR rate (50%) in previously untreated patients owing to greater immunocompetence. Consequently, we initiated a phase 2 clinical trial of upfront sequential PEM and AVD (Adriamycin, Vinblastine, Dacarbazine) chemotherapy. While several of the initial patients had dramatic responses to PEM x's 3 on PET2, they only met Lugano criteria for partial responses. In an attempt to better reflect and quantify the response to checkpoint inhibition, we amended our protocol to include additional analysis of metabolic tumor volume (MTV) and total lesion glycolysis (TLG) which have been investigated as predictors of response in cHL and DLBCL. Herein, we report an interim analysis of toxicity and efficacy, measured by Deauville score, MTV and TLG in the first 13 patients to complete PEM monotherapy.
Methods:
Patients ≥ 18 years of age with newly diagnosed cHL stages I-IV, including unfavorable early stage disease with at least one risk factor according to the NCCN criteria, were eligible. Patients had a pre-therapy PET-CT, followed by 3 cycles of PEM at 200 mg every 3 weeks and interim PET-CT (PET2) after single agent PEM for primary analysis. Subsequently, patients received 4-6 cycles of AVD chemotherapy based on initial stage. MTV representing the total volume of the metabolically active tumor in a volume of interest (VOI) was calculated using the fixed 41% SUVmax threshold corresponding to the dimensions of the tumor. TLG, combining the volumetric and metabolic information of FDG-PET, was calculated by multiplying the SUVmean of the segmented VOI and the MTV. These measurements were performed using SyngoVia software, Multi-foci segmentation tool (Siemens). Blinded central review of the visually assessed Deauville score was performed, and if different from the report by one of four nuclear medicine radiologists, a second central review was performed to adjudicate. Correlative studies include serum and biopsy samples pre/post PEM to assess immune biomarkers of response.
Results:
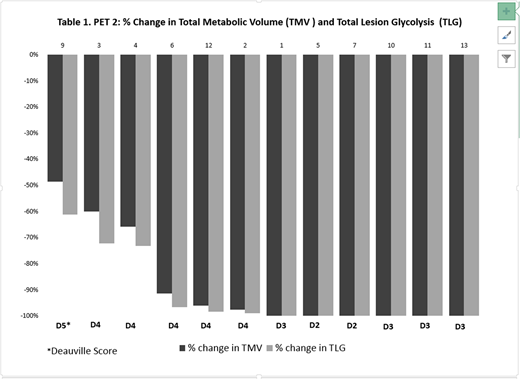
Fifteen patients were enrolled from September 2017, through a data cut off of July 10, 2018, at Northwestern University. Thirteen patients have completed lead-in PEM monotherapy and have undergone PET2. Median age was 30 years (range, 23-77). Eight patients had unfavorable early stage disease and 7 had advanced stage. Early stage risk factors included bulky disease (n=8), B-symptoms (n=5), and elevated ESR (n=5). Overall, therapy was well tolerated. Grade 3 events included lymphopenia (n=1) and diarrhea (n=1). Only one grade 4 immune-related adverse event (transaminitis) occurred, which resolved with steroid therapy and a delay in therapy. PET2 was scored as Deauville 2 or 3 in six cases including three with large mediastinal masses, and as D 4 or 5 in 7 cases with residual activity (Figure 1). PET2 MTV and TLG could be calculated for 12 of 13 cases, (MTV: 42 - 774 cm2, median 134 cm2; TLG: 257 - 5924; median 814). All 12 patients who completed a subsequent scan (PET3) after 2 cycles of AVD are now in a metabolic CR (Deauville 1-3), including all 7 cases with residual activity after PET2.
Conclusion: These interim data suggest that upfront PEM x's 3 is highly active in previously untreated cHL. PEM monotherapy resulted in complete metabolic responses in some patients including those with large mediastinal masses, and near complete responses, best represented by the decline in MTV and TLG compared with Deauville scores or Lugano criteria. Our preliminary data suggest that MTV and TLG are useful additional interim indicators of biologic activity when assessing response after PD-1 inhibitor therapy.
Allen:Merck: Research Funding; Bayer: Consultancy. Evens:Bayer: Consultancy; Janssen: Consultancy; Affimed: Consultancy; Novartis: Consultancy; Tesaro: Research Funding; Abbvie: Consultancy; Seattle Genetics, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy; Pharmacyclics International DMC: Membership on an entity's Board of Directors or advisory committees. Pro:kiowa: Honoraria; Takeda Pharmaceuticals: Honoraria, Other: Travel expenses; portola: Honoraria; Seattle Genetics: Consultancy, Other: Travel expenses, Research Funding. Advani:Millenium: Other: Institutional Research Support; Merck: Other: Institutional Research Support; Kura: Other: Institutional Research Support; Agensys: Other: Institutional Research Support; Takeda: Other: Consultancy/Advisory Role; Roche/Genentech: Other: Consultancy/Advisory Role, Institutional Research Support; Pharmacyclics: Other: Institutional Research Support; Cell Medica: Other: Consultancy/Advisory Role; Kyowa: Other: Consulting/Advisory Role; Janssen Pharmaceutical: Other: Institutional Research Support; Autolus: Other: Consultancy/Advisory Role; Regeneron Pharmaceuticals, Inc.: Other: Institutional Research Support; Seattle Genetics: Other: Consultancy/Advisory role, Institutional Research Support; Bayer Healthcare Pharmaceuticals: Other: Consultancy/Advisory Role; AstraZeneca: Other: Consultancy/Advisory Role; Bristol Myers Squibb: Other: Consultancy/Advisory role and Institutional Research Support; Forty Seven, Inc: Other: Institutional Research Support; Celgene: Other: Institutional Research Support; Gilead/Kite: Other: Consultancy/Advisory Role; Infinity: Other: Institutional Research Support. Winter:Merck: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal